Download the PDF File HERE
Arbaz Sajjad BDS, MDS
Specialist, Maxillo-Facial Prosthetic Clinic, Department of Prosthodontics, Specialist Dental center, Al-Jouf, Ministry of Health, KSA.
CORRESPONDING AUTHOR’S PERMANENT ADDRESS:
#179, 6th cross, BDS Nagar,K. Narayanapura, Bangalore- 560077, India. Phone:+966590362979.
Email: [email protected]
Keywords: Prosthetic Eyes, Prostheses, Impression Techniques, Eye Prostheses, Ocular Prosthesis, Ocular Conformer, Enucleation, Ocular implants, Custom- made acrylic eyes.
Abstract
Physical defects that compromise appearance or function, which prevents an individual from leading a normal life, usually prompt the individual to seek treatment that will reinstate acceptable normalcy. Throughout history, the human eye has been mentioned by authors as the most precious of gifts. It unveils the entire outer world to our consciousness, gives life, expression and dignity to the face. The loss of an eye therefore has always been regarded as the greatest misfortune and requires early replacement so that the patient may return to a normal life. An ocular prosthesis is a simulation of human anatomy using prosthetic materials to create the illusion of a perfectly normal healthy eye and surrounding tissues. Therefore much stress is given to the accurate duplication of colour, contour and size which will provide realism and symmetry for patients.
Introduction:
Eyes are generally the first features of the face to be noted1. The unfortunate loss or absence of an eye may be caused by a congenital defect, irreparable trauma, tumor, a painful blind eye, sympathetic ophthalmia or the need for histological confirmation of a suspected diagnosis2. The disfigurement associated with loss of an eye can cause significant physical and emotional problems3. Most patients experience significant stress, due primarily to adjusting to the functional disability caused by the loss and to societal reactions to the facial impairment 4. Replacement of the lost eye by an ocular prosthesis as soon as possible is necessary to promote physical and psychological healing for the patient and to improve social acceptance. An ocular prosthesis is a simulation of human anatomy using prosthetic materials to create an illusion of a perfectly normal healthy eye and surrounding tissue as well as to maintain the volume of the eye socket.
History:
The earliest known evidence of the use of ocular prosthesis is that of a woman found in Shahr-I Sokhta, Iran dating back to 2900-2800 BCE.5 It has a hemispherical form and a diameter of just over 2.5 cm (1 inch). It consists of very light material, probably bitumen paste. The surface of the artificial eye is covered with a thin layer of gold, engraved with a central circle (representing the iris) and gold lines patterned like sun rays. Roman and Egyptian priests are known to have produced artificial eyes as early as the fifth century BC constructed from painted clay attached to cloth and worn outside the socket.6Artificial eyes were constructed of such varied materials as gold, rock crystal, shell and colored stones. Ambrose Pare (1510-1590), a famous French surgeon, described the use of artificial eyes to fit an eye socket. These pieces were made of gold and silver, and two types can be distinguished: ekblephara and hypoblephara, intended to be worn in front of or under the eyelids, respectively. A hypoblephara eye (Figure-1) was designed to be used above an atrophic eye, as enucleation was not a common practice until the middle of the 1800’s. Pare later fabricated artificial eyes are made of glass as well as porcelain.7 8 9The first in-socket artificial eyes were made of gold with colored enamel, later evolving into the use of glass (thus the name “glass eye”). The glass eye originally came from Greeks of Dalmatia after the Latin war. From Greece, the knowledge travelled to Venice in the later part of the 16th century. These were crude, uncomfortable, and fragile and the production methodology remained known only to Venetians until the end of the 18th century, when Parisians took over as the center for artificial eye-making But the center shifted again, this time to Germany because of their superior glass blowing techniques. A German glass blower, Ludwig Muller —Uri, is credited with the development of fine artificial glass eyes.9 During the World War lithe supply of glass eyes from Germany to USA was halted. It was then that the Naval Dental School (USA) in 1943 tested the use of acrylic resin in fabricating ocular prosthesis.10
Pares-hypoblephara-rosthetic-artificial-eye
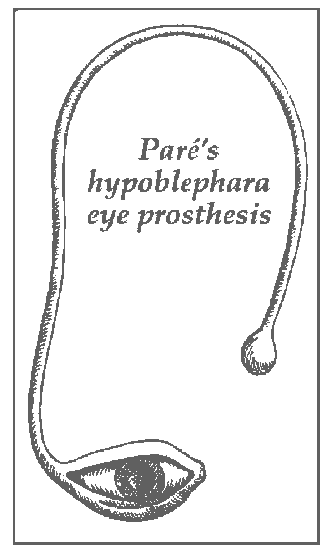
Figure -1. hypoblephara eye designed to be used above an atrophic eye.
Applied anatomy of the eye:
The eyeball occupies only the anterior part of the orbit. The globe of the eye is a slightly asymmetrical sphere somewhat flattened ii-om above downwards. The adult eyeball measures about 2.5cm (one inch) in diameter. The globe is widest at its antero-posterior diameter (24mm) and is flattened from above downwards.” The comeoscleral envelope is fibrous and anterior sixth is perfectly transparent, while its posterior five-sixths, the sclera is white and opaque. The cornea is a transparent coat that covers the colored iris.
Surgical considerations:
The surgical procedures in the removal of an eye are classified into —
1) Evisceration
It involves the removal of the contents of the globe leaving in place the sclera and sometimes the cornea. A loss of volume results from its removal. The mobility of the eviscerated globe implant is excellent, since the extra ocular muscles are intact. The prosthesis best suited is the custom cosmetic cover shell or the sclera cover shell prosthesis. A minimum of one mm thickness is required. Most patients remove the sclera shells at night since the remaining globe is usually very sensitive.12
2) Enucleation
It is the surgical removal of the eyeball after the eye muscles and the optic nerve has been severed. Adequate space is created for fabricating the ocular prosthesis. It is the movement of the fornix in the enucleated socket that provides the mobility to the artificial eye.12
An ideal socket for the fitting of an ocular prosthesis should have:
1. A well placed implant with the extra ocular muscle attached.
2. Adequate superior and inferior furnaces for positive retention of the prosthesis.
3. A palpebral fissure equal in size and shape to the tissues of the natural eye.
4. Adequate anterior-posterior depth to the socket.
5. Adequate support of the superior and inferior tarsal plates.
6. Minimum scar tissue adhesions in the socket.
7. Adequate mobility of the eyelids.
8. Some type of tissue irregularities in the depth of the socket for the positive adaptation of the prosthesis.
A contracted socket with inadequate superior and inferior fornices, with palpebral fissures of unique size and shape and with inadequate anterior- posterior socket depth presents with numerous retention and cosmetic complications. Prosthetic treatment of a contracted socket involves the construction of sequentially larger pressure conformers to expand and shape it.
3) Exenteration
It is the removal of the entire contents of the orbit, including the extra ocular muscles. The eyelids may or may not be involved. Exenteration defects in some instances may be allowed to heal by secondary intent but adequate space must remain in the resultant defect to allow the prosthesis to be positioned superiorly and posteriorly enough for a good cosmetic appearance.12
Clinical considerations:
A patient’s history including the details of the nature of the disease, its mode of onset with reference to the visual status and recurrence should be taken. Family history is important, congenital or hereditary anomalies such as cataract, albinism and iris deformities etc.9
During the post-operative period, it is important that the patient wore a conformer. The presence of a conformer will aid in the preservation of cul-de-sac of the fornices and in stabilization of the implant during healing. The construction of a custom conformer may be indicated when the construction of a definitive ocular prosthesis will be delayed because of slow patient recovery, medical complication or patient preference9.The socket is examined to determine the presence of an implant and the degree of mobility. Mobility may be noted by observing the movement of the tissue bed when the natural eye moves. If the patient has previously worn any prosthesis, the type, tolerance and difficulties if any, experienced are also noted.14 A growing child will require periodic enlargement of the prosthesis gradually over a period of years to aid in the development of the lids and other soft tissues lining the orbital bone margins which must be stretched to enhance the development of the fornices, which is necessary for good cosmetic result. The amount of orbital adipose tissue present and the extent of atrophy of muscle and other tissues incident to the removal of the eye, as well as the contour and tonus of the eyelids, should be particularly evaluated at the time of examination.8
Various types of artificial eyes:
a) Based on the material used for fabrication: Glass eyes
It is a combination of fusible opaque glass for sclera portion and transparent glass for corneal portion. The opaque is obtained from a combination of 30% silicone and 20% potassium and 30% lead oxide and 10% tin oxides. The transparent corneal glass is obtained by merely omitting the metal oxide. Glass eyes are rarely used because of the difficulty in handling and adjusting the material. They are useful in cases of allergy to resin.
Acrylic resin eyes. Developed in 1939 by the armed forces of the United States and it makes use of poly methyl methacrylate (PMMA). It is compatible with tissues, is easy to work with, costs less than glass, and has easy color modification abilities and more aesthetic appearance than glass.
b) Based on the Fit:
Custom eyes
Are individually constructed, hand painted acrylic resin artificial eyes. It, however, necessitates the service of a skilled artist for the iris and sclera, and is an involved and time-consuming process15
The optimum cosmetic and fuctional results enhance the patients’ rehabilitation to a normal life style.
Stock eyes
Developed by commercial eye optical companies. The procedure may be done in a very shol1period of time; but the esthetic and functional results were not satisfactory. It may be used as interim prosthesis, as a conformer or stent immediately, post operatively to aid in the regrowth and orientation of the tissues in surgical area 16.
diagram-artificial-eye-prosthetics-oculoplastic-surgery-ophthalmologist-ocularist-eye-maker-ocularist
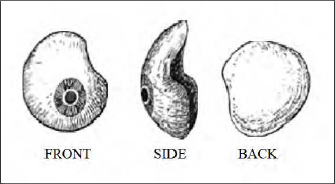
The Most commonly used was the Snellen conventional reform eyes (Figure -2).
Variations in the reform eye:
1) Conventional shell type:
Indicated where orbital tissues are protuberant and leave too little space for a reform eye. The thickness of the scleral portion is about 1-1.5mm
2) Hook or shelf type:
It is indicated in the eyes with the shallow lower fornix and lax lower lid which leads to a tendency for the prosthesis to slip out from below. A hook at 90o supports the prosthesis by resting over the stump taking away some weight being exerted on the lower lid.
3) Curled back shell:
The upper portion of prosthesis itself extends back at right angle to the vertical fold of the eye. Indicated in cases of shallow or deficient inferior fornix.
4) Forty five degree bent eye:
When an ordinary reform eye would lean back at an angle of 45° from the horizontal, the band prevents drooping of the temporal portion of the upper eyelid.
5) Peanut eye:
These eyes, shortened in vertical direction and elongated horizontally with a temporal curve are used when a conventional reform eye tends to sink back temporarily and pulling away from the inner canthus.
6) Reversed shape:
The vertical dimension is greater nasally and temporally. It is used when the prosthesis has a tendency to rotate in the socket.
Impressions Techniques for custom ocular Prosthesis:
1. External tray impression technique:
As the name suggests in this technique impression of the anopthalmic socket in conjunction with the surrounding supporting anatomical structure is made with the aid of an external impression tray. Several authors have used a technique in which low viscosity alginate or reversible hydrocolloid is injected directly into the enucleated socket 17 18. The patient is instructed to stare straight ahead with his gaze fixed at a point 6 feet away in the line of vision as the material sets. Additional material is applied to the external tissue, and an impression is made using a perforated acrylic resin backing tray loaded with alginate placed over the defect. The impression is boxed, poured in dental stone up to the height of contour of the impression. A separating agent is applied and the impression is poured in type IV gypsum after making at least two keyways. Thus a 2-piece split cast is obtained.
2. Molded shell/ stock tray impression technique:
It is perhaps the most commonly followed impression technique which was first described by Allen and Webster 19.In this technique is made only of the anopthalmic socket using impression trays shaped like a stock ocular prosthesis. The patient is always seated upright with head rest. These trays are made of acrylic resin have perforations that aid in the flow and retention of the impression material and have a hollow handle which accommodates a plastic disposable impression syringe. Ophthalmic grade irreversible hydrocolloid is injected into the socket via the impression syringe through the hollow tube of the impression tray. After it has set, the impression is removed and rinsed in the water and replaced back in the defect to check for proper lid contour and mobility of the impression. Subsequently, the impression is removed and invested in type IV gypsum ( Figure-3).
Figure-3. Molded shell/ stock tray impression
Variations of the stock tray impression technique:
Variations of the stock tray impression technique center on the design and materials used in the fabrication of the stock ocular tray. Maloney20 placed 3 channels through the superior edge of his own set of customized stock trays to prevent air entrapment. Following his method, a raised ring around the stem prevents the eyelid from blocking the channels. Engelmeier21 suggested casting a set of stock trays in Ticonium to permit Sterilization and reuse. Sykes et al 22 advocated the use of modeling plastic impression compound as an ocular tray material, forming it around one half of a small rubber ball and placing a hollow tube through it.
3. Impression technique using a stock ocular prosthesis:
The use of a stock ocular prosthesis of an appropriate size and color, adapted by selective grinding or addition of acrylic resin has been advocated by Laney and Gardener23.A stock eye is selected with the correct iris size, color and sclera shape. The periphery and posterior surface is reduced 2-3mm and retentive grooves are cut into it. Alginate adhesive is painted over these surfaces and alginate is injected into the defect and the modified stock eye is placed into it. Impression is then invested, packed and cured under 3500 psi pressure for at least one hour. Limitations of this technique include the need to maintain a fairly large supply of artificial eyes and the inability to match all sizes and colors of the iris and pupil.
Variations of the stock ocular prosthesis impression technique:
Modification of stock eye prosthesis can also be done using a tissue conditioner as described by Ow and Amrith24. This is comfortable and produces a healthy clinical soft tissue response. Its biocompatibility allows the continued clinical use and evaluation of the ocular prosthesis over an extended period of 24 to 48 hours. This method is particularly suitable in growing children where the prosthesis needs to be regularly modified to suitably fit their growing orbits. After 48 hours, the elastic tissue conditioner must be converted into heat cured acrylic resin to complete the prosthesis. Smith described a reline procedure for an existing prosthesis using a dental impression wax such as Korecta-Wax No. 4 (D-R Miner Dental, Orinda, CA).25 For definitive refinement, the lined prosthesis is left in place for 30 minutes while the patient intermittently moved his or her eyes in all directions.
Ocular Implants:
When an eye is removed, an orbital implant is used to replace the volume in the orbit that was occupied by the eye. The implant itself which is small & spherical is not visible and placed in the tissue bed facilitates construction of an ocular prosthesis thus preventing sunken appearance of the orbit. An implant that is attached to the ocular muscles move in their normal course, consequently the prosthetic eye will exhibit some degree of movement). Also, in growing children, the restored muscle function creates tension in the orbital walls and ensures a normal pattern of orbital growth. There are many different types of implants, classification ranging from shape (Spherical vs. egg (oval) shaped), stock vs. custom, porous vs. non porous, specific chemical make-up, and the presence of a peg or motility post. The most basic simplification can be to divide implant types into two main groups: nonintegrated (non-porous) and integrated (porous)26.
Nonintegrated implants (Non Porus):
The nonintegrated spherical intraconal implants came into existence around 1976 (not just glass eyes. They contain no unique apparatus for attachments to the extraocular muscles and do not allow in-growth of organic tissue into their inorganic substance. Such implants have no direct attachment to the ocular prosthesis26. Usually, these implants are covered with a material that permits fixation of the extraocular recti muscles, such as donor sclera or polyester gauze which
improves implant motility, but does not allow for direct mechanical coupling between the implant and the artificial eye. Non-integrated implants include poly methylmethacrylate (PMMA), glass, and silicone spheres.
Polymethyl methacrylate (PMMA):
PMMA has a good degree of compatibility with human tissue, much more so than glass. Although various materials have been used to make nonintegrated implants in the past, PMMA is one of the implants of choice26.
Integrated implants (porous):
The porous nature of integrated implants allows fibrovascular ingrowth throughout the implant and thus also insertion of pegs or posts. Because direct mechanical coupling is thought to improve artificial eye motility, attempts have been made to develop so-called ‘integrated implants’ that are directly connected to the artificial eye27. In 1985, spherical implants made of porous calcium hydroxyapatite (HA) were introduced. Porous enucleation implants currently are fabricated from a variety of materials including natural and synthetic hydroxyapatite, aluminum oxide, and polyethylene.
Hydroxyapatite (HA):
Since their introduction in 1989 when an implant made from HA received Food and Drug Administration approval, spherical HA implants have gained widespread popularity as an enucleation implant 27 and were at one point was the most commonly used orbital implant in the United States. The porous nature of this material (Figure-4) allows fibrovascular in growth throughout the implant and permits insertion of a coupling device (PEG) with reduced risk of inflammation or infection associated with earlier types of exposed integrated implants27. However one main disadvantage of HA is that it needs to be covered with exogenous material, such as sclera, polyethylene terephthalate, or vicrylmesh (which has the disadvantage of creating a rough implant tissue interface that can lead to technical difficulties in implantation and subsequent erosion of overlying tissue with the end stage being extrusion), as direct suturing is not possible for muscle attachment. Scleral covering carries with it the risk of transmission of infection, inflammation, and rejection.

Figure-4. Porous Hydroxyapatite (HA) implant.
Porous polyethylene (PP):
Development in polymer chemistry has allowed introduction of newer biocompatible material such as porous polyethylene (PP) to be introduced into the field of orbital implant surgery. Porous polyethylene enucleation implants have been used since at least 1989. It is available in dozens of prefabricated spherical and non-spherical shapes and in different sizes or plain blocks for individualized intraoperative customizing. The material is firm but malleable and allows direct suturing of muscles to implant without wrapping or extra steps. Additionally, the smooth surface is less abrasive and irritating than other materials used for similar purposes. Polyethylene also becomes vascularized, allowing placement of a titanium motility post that joins the implant to the prosthesis in the same way that the peg is used for hydroxyapatite implants28.
Bioceramic:
Bioceramic prosthetics are made of aluminum oxide (Al203). Aluminum oxide is a ceramic biomaterial that has been used for more than 35 years in the orthopedic and dental fields for a variety of prosthetic applications because of its low friction, durability, stability, and inertness. Aluminum oxide ocular implants can be obtained in spherical and non-spherical (egg-shaped) shapes and in different sizes for use in the anophthalmic socket28. Aluminum oxide has previously been shown to be more biocompatible than HA. The rate of exposure previously associated with the bioceramic implant (2%) was less than most reports on the HA or porous polyethylene implant (0% to 50%)29.
Dermis-fat orbital implantation:
The use of dermis fat graft to reconstruct an anophthalmic socket was first described by Smith and Petrelli in 1978. The use of dermis fat graft as a primary orbital implant after enucleation and as a secondary implant following exposure or extrusion of alloplastic implants has also been reported.30 Dermis fat grafts have the advantages of relative abundancy and light weight. For the same volume, a dermis fat graft is lighter than silicone or hydroxyapatite implants. Previous reports have documented the harvest of dermis fat from the gluteal area, hip region, inner thigh, and arm.31 The dermis fat graft is harvested as a cylindrical shape with the fat portion flared out so that the diameter of the fat is slightly larger than the diameter of the dermis cap. The elongated cylindrical shape of the fat allows for an overfilled volume within the eviscerated scleral shell as fat atrophy is anticipated. Patients that are at risk for alloplastic implant exposure from poor wound healing may benefit from this procedure. Due to its high degree of safety concurrent with excellent functional and cosmetic results, the dermis-fat transplant is particularly advantageous for young patients.32
Pegged (motility post) implants:
In hydroxyapatite and polyethylene implants a secondary procedure can insert an externalized, round-headed peg or screw into the implant that fits into a corresponding dimple at the posterior surface of the artificial eye (Figure-5). This peg thus directly transfers implant motility to the artificial eye. However, the motility peg is mounted in only a minority of patients. This may partially be the result of problems associated with peg placement, whereas hydroxyapatite implants are assumed to yield superior artificial eye motility even without the peg27.
Figure-5. Titanium Pegged (motility post) implant kit
Surgical Procedure:
The surgery is done under general anesthesia with the addition of extra subconjunctival and/or retrobulbar anesthetics injected locally in some cases. The following is a description of the surgical procedure performed by Custer et al 28:
The conjunctival peritomy is performed at the corneal limbus, preserving as much healthy tissue as possible. Anterior Tenon’s fascia is separated from the sclera. Blunt dissection in the four quadrants between the rectus muscles separates deep Tenon’s fascia. Sutures may be passed through the rectus muscles before their disinsertion from the globe. Some surgeons also suture one or both oblique muscles. Traction sutures or clamps are applied to the horizontal rectus muscle insertions to assist in rotating and elevating the globe during the ensuing dissection. Tenon’s capsule is opened posteriorly to allow visualization of the optic nerve. The vortex veins and posterior ciliary vessels are cauterized before dividing the nerve and removing the eye. The orbital implant is inserted at the time of enucleation. An appropriately sized implant should replace the volume of the globe and leave sufficient room for the ocular prosthesis. Enucleation implants are available in a variety of sizes that may be determined by using sizing implants or calculated by measuring globe volume or axial length of the contralateral eye. Tenon’s fascia is drawn over the implant and closed. The conjunctiva is then sutured. A temporary ocular conformer is inserted at the completion of the procedure and is worn until the patient receives prosthesis 4 to 8 weeks after surgery. An elective secondary procedure is required to place the coupling peg or post in those patients who desire improved prosthetic motility. That procedure is usually delayed for at least 6 months after enucleation to allow time for implant vascularization. Technetium bone or gadolinium-enhanced magnetic resonance imaging scans are not now universally used to confirm vascularization before peg insertion. Under local anesthesia, a conjunctival incision is created at the peg insertion site. A hole is created into the porous implant to allow insertion of the peg or post. The prosthesis is then modified to receive the peg or post.
Discussion:
Throughout history, the human eye has been mentioned by authors as the most precious of gifts. It unveils the entire outer world to our consciousness, gives life, expression and dignity to the face. The loss of an eye therefore has always been regarded as the greatest misfortune and requires early replacement so that the patient may return to a normal life. The art and science of ocular prosthesis has been refined over many decades to provide a cosmetic replacement of the enucleated or eviscerated eye. The fabrication of a definitive ocular prosthesis should begin as soon as the socket has healed. A correctly placed prosthesis should restore the normal opening of the eye, support the eyelids, restore a degree of movement, and be adequately retained and esthetically pleasing. The use of a stock ocular prosthesis of an appropriate size and color, adapted by selective grinding or addition of acrylic resin, has been advocated by Laney and Gardner23. Another similar technique involves the application of a viscoelastic tissue conditioner material as an impression material to modify a stock ocular prosthesis in relation to an anophthalmic socket24. However because of extreme individual variation and diverse nature of ocular injuries, certain patients would benefit more from custom made ocular prostheses that are modified to their individual needs. This procedure may be more time-consuming and entail a “trial and error” approach, but the esthetic and functional results justify the extra effort. Till date various materials like gold, silver, glass, acrylic & even porcelain 7 8 9 have been used for making artificial eyes, but the preferred material is acrylic. The material is lightweight, easy to fit and adjust, unbreakable, translucent, easily fabricated, has intrinsic and extrinsic coloring capabilities, and is inert to the socket secretions. Prosthetic rehabilitation is enhanced if an implant can be placed in the orbit to provide an attachment for the rectus muscles, which can impart motion coordinated with the natural eye. The goal is to return the patient to the society with a normal appearance and reasonable motility of the prosthetic eye.
Conclusion:
Loss of any part of the face inflicts both physical and psychological trauma to the patient. The disfigurement resulting from loss of an eye can cause significant psychological as well as social consequences. A thorough knowledge of the regional anatomy and recent developments in the field is prudent. One needs to be a little artistic and very innovative to treat such patients, utilizing the available materials and techniques. Above all empathy towards the patient’s condition must be present.
References:
1. Doshi P, Aruna B. Prosthetic management of patient with ocular defect. Journal of
Indian Prosthodontic Society.2005; 5(1):37-8.
2. Perman KI, Baylis HI. Evisceration, enucleation, and exenteration. Otolaryngol
Clin North Am.1988; 21(1):171-82.
3. Lubkin V, Sloan S. Enucleation and psychic trauma. Advances in ophthalmic plastic and reconstructive surgery.1990; 8:259.
4. Artopoulou I, Montgomery P, Wesley P, Lemon J. Digital imaging in the fabrication of ocular prostheses. J Prosthet Dent. 2006; 95(4):327-30.
5. Wikipedia.org [homepage on the Internet]. Wikipedia, the free encyclopedia:Ocular
Prosthesis 2011 [ Cited 2012 Feb 13]. Available from:
http://en.wikipedia.org/wild/Ocular_prosthesis.
6. Fox News [homepage on the Internet]. Resource London Times 2007 [Cited 2012 Feb
13]. Available from: http://www.foxnews.com/story/0,2933,253221,00.html
7. Beumer J, Curtis TA, Firtell DN. Maxillofacial rehabilitation: Prosthodontic and surgical consideration. St. Louis: C.V.Mosby Co.; pp.348-371.
8. Chalian VA, Drane JB, Standish SW. Maxillofacial prosthetics: Multidisciplinary practice. Baltimore: Williams and Wilkins; pp.286-294.
9. Smith BC, Della Rocca RC, Nesi FA. Ophthalmic plastic and reconstructive surgery, vol
II. St. Louis: C.V. Mosby Co.; pp.1321-1328.
10. Sykes LM. Custom made ocular prostheses: A clinical report. J Prosthet Dent. 1996;
75:1-4.
11. Bron AJ, Tripathi RC. Wolff s anatomy of the eye and orbit, 8th ed. Spain: Chapman and
Hall; pp 30-32 and 211-212.
12. Parr GR, Goldman BM, Rahn AO. Surgical considerations in the prosthetic treatment of ocular and orbital defects. J Prosthet Dent. 1983; 49:379-385.
13. Kumar D, Krishna G. Cosmetic contact lenses and artificial eyes. J Contact Lens
Research and Training Institute
14. Cain JR. Custom ocular prosthetics. J Prosthet Dent. 1982; 48:690-694.
15. Tortora Grabowski. Principles of anatomy and physiology, 10th ed. Wiley International
Edition; pp 531- 36.
16. Welden RB, Niiranen JV. Ocular prosthesis. J Prosthet Dent. 1956; 6:272-278.
17. Bartlett S, Moore D. Ocular prosthesis: A physiologic system. J Prosthet Dent. 1973;
29(4):450-9.
18. Brown K. Fabrication of an ocular prosthesis. J Prosthet Dent. 1970; 24(2):225-35.
19. Allen L, Webster H. Modified impression method of artificial eye fitting. American journal of ophthalmology1969; 67(2): 189-218.
20. Maloney B. Development of impression fitting equipment: A new technique. J Am Soc
Ocularists.1979; 9:32-3.
21. Engelmeir RL. Autoclavable custom made metal impression trays to improve infection control. J Prosthet Dent. 1987; 58: 121-2.
22. Sykes L, Essop A, Veres E. Use of custom-made conformers in the treatment of ocular defects. J Prosthet Dent. 1999; 82(3):362-5.
23. Laney WR, Gardner AF. Maxillofacial prosthetics. PSG Publishing Company: Littleton, Massachusetts; 1979: pp. 284-286.
24. Ow R, Amrith S. Ocular prosthetics: use of a tissue conditioner material to modify a stock ocular prosthesis. J Prosthet Dent. 1997; 78(2):218-22.
25. Smith R. Relining an ocular prosthesis: A case report. J Prosthodont. 1995; 4:160-3.
26. Shome, D; Honavar, SG; Raizada, K; Raizada, D.”Implant and prosthesis movement
after enucleation: a randomized controlled trial”. Ophthalmology. 2010; 117 (8):
1638-44.
27. Colen, TP; Paridaens, DA; Lemij, HG; Mounts, MP; Van Den Bosch, WA. “Comparison of artificial eye amplitudes with acrylic and hydroxyapatite spherical enucleation implants”. Ophthalmology. 2000; 107 (10): 1889-94.
28. Custer, PL; Kennedy, RH; Woog, JJ; Kaltreider, SA; Meyer, DR. “Orbital implants in enucleation surgery: a report by the American Academy of Ophthalmology”.0phthalmology. 2003; 110 (10): 2054-61.
29. Jordan, DR; Klapper, SR; Gilberg, SM; Dutton, JJ; Wong, A; Mawn, L.”The bioceramic implant: evaluation of implant exposures in 419 implants”. Ophthalmic plastic and reconstructive surgery. 2010; 26 (2): 80-2.
30. Smith B, Petrelli R. Dermis-fat graft as a movable implant within the muscle cone. Am
J Ophthalmol 1978; 85:62-6.
31. Hintschich CR, Beyer-Machule CK.Dermal fatty tissue transplant as primary and secondary orbital implant. Complications and results. Ophthalmologe. 1996;
93(5):617-22.
32. Tarantini A, Hintschich C. Primary dermis-fat grafting in children. Orbit. 2008;
27(5):363-9.
